- Review
- Open access
- Published:
The war between the immune system and the tumor - using immune biomarkers as tracers
Biomarker Research volume 12, Article number: 51 (2024)
Abstract
Nowadays, immunotherapy is one of the most promising anti-tumor therapeutic strategy. Specifically, immune-related targets can be used to predict the efficacy and side effects of immunotherapy and monitor the tumor immune response. In the past few decades, increasing numbers of novel immune biomarkers have been found to participate in certain links of the tumor immunity to contribute to the formation of immunosuppression and have entered clinical trials. Here, we systematically reviewed the oncogenesis and progression of cancer in the view of anti-tumor immunity, particularly in terms of tumor antigen expression (related to tumor immunogenicity) and tumor innate immunity to complement the cancer-immune cycle. From the perspective of integrated management of chronic cancer, we also appraised emerging factors affecting tumor immunity (including metabolic, microbial, and exercise-related markers). We finally summarized the clinical studies and applications based on immune biomarkers. Overall, immune biomarkers participate in promoting the development of more precise and individualized immunotherapy by predicting, monitoring, and regulating tumor immune response. Therefore, targeting immune biomarkers may lead to the development of innovative clinical applications.
Background
As the third revolution in the history of drug therapy for malignant tumors, immunotherapy is regarded as one of the most promising anti-tumor therapies at present [1]. According to the immune surveillance theory, immunotherapy, which differs from conventional radiotherapy, chemotherapy, and targeted therapy, can leverage the anti-tumor immune system of patients to recognize and eliminate foreign tissues, such as tumors [2]. Additionally, it plays a crucial role in maintaining the equilibrium stage or potentially restoring the clear stage through reversing the escape stage, as per the tumor immunoediting theory, thereby effectively managing tumor progression over an extended period [3]. Due to its high universality, good effect on responders and low side effects, immunotherapy is no longer just a late-line treatment for patients with advanced tumors, but has gradually become the main means of tumor treatment, actively used in adjuvant or neoadjuvant therapy, and combined with other anti-tumor drugs such as targeting or anti-angiogenesis [4]. Due to the high side effects of chemotherapy drugs and low patient compliance, immunotherapy is gradually replacing chemotherapy drugs in front-line treatment in advanced lung cancer, leding to a new era of “chemo-free” treatment [5]. In addition, with the advancement of immunological knowledge and biological techniques, the emergence of “synthetic immunology” (primarily involving artificial manipulation and the creation of human immunity) has contributed to making immunotherapy more precise and effective [6].
However, there are still problems in immunotherapy: few patients respond to immunotherapy, and many of them have side effects mainly caused by drug resistance and immune-related adverse reactions (IRAEs). Meanwhile, the research of immunotherapy drugs is also mired in difficulties due to the lack of suitable targets [7]. In order to solve the above problems, we need to deeply study all aspects of immunogenesis and development of tumor to explore more suitable immune biomarkers as targets for monitoring and treatment.
Tumor immune biomarkers are biological features or indicators that can be objectively measured and used to evaluate tumor immune progression [8]. It is used to predict the efficacy and side effects of immunotherapy, monitor the immune response of tumors and provide targets for related drug research. Then we can screen out patients who are more suitable for immunotherapy, and may even make non-responders turn to responders or transform “cold tumors” to “hot tumors“ [9]. The treatment based on immune biomarkers breaks the concept of unified standardized treatment according to different tumor sources in the past, but carries out precise individual treatment according to the expression of markers [10]. In fact, various molecules (like receptors or cytokines), cells (of different types and proportions), microstructures (like tertiary lymphoid structures (TLS)), and even pathological features (like tumor cell death) involved in each stage of tumor immunity can be used as potential markers. However, attention should be paid to the extent of its contribution to the overall tumor immunity and the conversion possibility of clinical application [11]. Interestingly, in addition to naturally occurring ones during tumor immunity, biomarkers can also be artificially induced. For example, genetically engineered bacteria can specifically infect tumors while expressing corresponding antigens as biomarkers for immune cells to recognize tumors, and even produce substances similar to immune checkpoint inhibitors (ICIs) [12]. In addition to the exploration of the biomarkers themselves, the acquisition and detection means of immune biomarkers, the design of drug structure and the way of administration also affect the clinical application value of markers to a large extent [13].
Therefore, in order to search for immune biomarkers with high tumor specificity, good efficacy, and convenient clinical application, we updated and refined the rate-limiting aspects of the cancer-immunity cycle related to markers based on the remarkable progress of the past decade, particularly in terms of tumor antigen expression (related to tumor immunogenicity) and tumor innate immunity. The treatment of cancer is now gradually entering the “era of chronic disease management.” In addition to individualized treatment based on biomarkers, we also described comprehensive treatment, which includes the patient’s diet, exercise, and lifestyle habits. Finally, we focused on the clinical application and research progress based on tumor immune biomarkers.
Tumor immunity and its markers
The heterogeneity of tumor (primary drug resistance, Darwinian natural selection) and its ability to adapt to survival pressures (secondary drug resistance, Lamarkian evolution) result in the failure of therapeutic strategies that act directly on the tumor due to adaptive changes in the tumor target [14]. No matter how the tumor mutates, it is considered foreign to the body, exhibiting the behavior and characteristics of abnormal cells. Therefore, regulating the patients’ own tumor immune response sthrough immune biomarkers to indirectly fight against the tumor is a therapeutic strategy of “responding to all changes with no changes”. However, there are two main ways for the tumor to suppress anti-tumor immunity to form “cold tumors”. On the one hand, tumors can disguise themselves as normal cells to reduce their immunogenicity and thus reduce the detection of the immune system. On the other hand, tumors arm themselves with immunosuppressive tumor microenvironment (TME) to impede immune effects [15].
Initiation of tumor immune response
Tumor antigens are the basic biomarkers for the immune system to recognize tumors
Tumor antigens are tumor biomarkers that are abnormally expressed with the development and progression of tumors, mainly due to abnormalities at the gene level, but also due to abnormalities in the antigen synthesis process, such as post-transcriptional RNA splicing disorder or post-translational protein modification disorder [16]. In addition, it takes time for mutations to accumulate in normal somatic cells until they affect genes crucial for cell proliferation and death, eventually leading to tumorigenesis. During this time, normal somatic cells can also produce tumor antigens due to mutations in tumor-related genes, and even induce anti-tumor immunity (similar to autoimmunity) in non-tumor situations [17].
On the one hand, when affected by external influences such as physical, chemical or biological carcinogenic factors, or by internal influences such as spontaneous DNA replication errors, the cell will have gene mutations, which belongs to classical genetics. It is mainly manifested as the imbalance between proto-oncogenes and tumor suppressor genes [18]. Changes in gene sequences cause tumor cells to produce antigens that normal cells do not express, namely tumor-specific antigens (TSAs, also known as neoantigens), with high immunogenicity and individual heterogeneity [19]. It is worth noting that the genetic mutations that cause carcinogenesis can be divided into a few key driver genes and a large number of accompanying passenger genes. Theoretically, the greater the number of mutations, that is, the higher the tumor mutation burden (TMB), the more TSAs will be produced, although not all mutations associated with TMB-H will enhance the antitumor immune response [20]. In addition, the quality of the mutation also affected the production of TSAs, The study found that TSAs mainly came from the mutation of non-driver genes, while the mutation of passenger genes has a weak effect on the fitness of tumor cells [21]. Mutations in the genome have been found to contribute to tumor growth in 5–10% of patients [22].
On the other hand, abnormal gene expression may occur in cells, It is mainly manifested in the differences in the timing, spatial distribution, and level of gene expression compared to normal physiological or non-cancerous pathological processes [23]. Because there is no genetic sequence change, tumor cells will produce antigens that normal cells can also express, that is, tumor-associated antigens (TAAs), which have weak immunogenicity due to the formation of immune tolerance. TAAs include cancerous testicular antigen, differentiated antigen, overexpressed antigen and cancerous embryo antigen [24]. Notably, some TAAs can also be derived from classical genetic pathways, such as gene recombination that reactivates silenced promoters. Unlike other TAAs, some carcinoembryonic antigens reexpressed later still have high immunogenicity because the expression of normal embryonic proteins precedes the formation of immune tolerance [25].
Different from TAA, TSA is more tumor specific and is key to initiating the body’s anti-tumor immunity and T cell effector activation [26]. However, tumor antigens are not only biomarkers of mediated immunity or anti-tumor drugs targeting tumors, some tumor antigens promote the occurrence and development of tumors to a large extent. For example, some breast cancer cells overexpress human epidermal growth factor receptor 2 (Her-2), which enhances cell proliferation and differentiation mediated by growth factor signaling [27]. Therefore, for tumors, the balance between the survival promotion effect of tumor antigens and the anti-tumor immunity they provoke determines the tumor immunophenotype, including sensitivity and resistance to immunotherapy. Overall, tumors tend to hide TSA and masquerade as normal cells by expressing TAA, mediating immune escape [28].
In addition to the antigens spontaneously produced in the above cellular carcinogenic pathways, foreign microbial antigens expressed by non-carcinogenic pathways, such as viruses and bacteria, can also be used as tumor antigens. Microbial antigens activate anti-microbial immunity while destroying host tumor cells [29]. According to the state of the infected cell, microbial antigens can be divided into two categories. The one type of antigens arise from the process in which normal cells get infected and transform into tumor cells. Most of them are only related to the early stage of tumor development, while the later stage of tumor progression is related to the carcinogenesis pathway it induces. Other normal cells will also produce the same antigens after being infected. The other type of antigens is expressed after specific infection of tumor cells, which are mostly expressed by genetically engineered bacteria [30]. In recent studies, human endogenous retroviruses (HERVs) have been found to be more than the remnants of ancient retroviruses with low transcriptional activity. They can be reactivated by several factors, such as environmental carcinogenic factors, which may drive the expression of oncogenes and activate anti-tumor innate immunity by inducing viral defense pathways [31].
Tumor cell death is a biomarker of the release of tumor internal antigens and the initiation of immune cell infiltration
Immune cells must be able to recognize and contact tumor antigens in order to initiate tumor immunity. The immune system isn’t able to actively detect tumors. For one thing, immune cells patrol and supervise the body’s cells, that is lymphocyte recirculation, to increase the chance of contact with tumor antigens. But the cells involved in this recirculation are mainly memory T and B cells, whose primary role is to maintain long-term immunity rather than to initiate initial immunity [32]. For another, tumor cells can hide tumor antigens by resisting senescence and death, reducing and modifying the expression of major histocompatibility complex (MHC), and gradually differentiate into less immunogenic phenotypes with the progress of tumor immunoediting [2].
However, the mismatch between uncontrolled tumor proliferation and local resource supply results in the lack of available resources and the accumulation of metabolic wastes, such as hypoxia, glucose deficiency, lactic acid accumulation and oxidative stress. It inevitably leads to tumor cell senescence, damage, such as endoplasmic reticulum (ER) stress, and non-apoptotic regulatory cell death (RCD), especially immunogenic cell death (ICD) [33]. The death of tumor cells will release tumor internal antigens, adenosine triphosphate (ATP) and high mobility group protein 1 (HMGB1) and other immune stimulators to activate the tumor immune response. Similarly, dying tumor cells can also express damage-associated molecular patterns (DAMPs), such as ER chaperone calreticulin (CRT/CALR) and heat shock protein (HSP), which attract and activate innate immune cells by binding to pattern recognition receptors (PRRs) [34]. In addition, senescent tumor cells also secrete a cocktail of proinflammatory cytokines to form the senescence-associated secretory phenotype (SASP), which in turn regulates TME. Changes in the proportion of senescent cells in tumors before and after treatment have been recognized as one of the hallmarks of cancer [35].
Therefore, the death or injury of tumor cells is an important biomarker for the release of tumor internal antigens and the initiation of immune invasion. It is often used as a clinical target to develop various therapeutic strategies for inducing non-apoptotic RCD in tumors, such as ICD inducers [36]. However, this does not explain why healthy tumor cells in early TME can be recognized by the immune system when resources are sufficient, especially before the immune system applies the selective pressure to screen for lower immunogenic phenotypes. In this regard, recent studies have found that differences in epigenetic modification of the RNA-binding protein cold shock domain-containing protein E1 (CSDE1) can lead to high or low immunogenic heterogeneity in early neonatal tumor cells [37].
However, the death or injury of tumor cells does not always initiate an anti-tumor immune response, and may even suppress tumor immunity. Since cell death often occurs inside the tumor tissue, it is possible that the ‘corpse’ of the tumor is buried by the TME and cannot be detected by immune cells [38]. Even if the immune infiltration is activated, most tumor cells can hide their own antigens and pretend to be ‘innocent bystanders’. At this time, a large number of infiltrated immune cells can act as ‘scavengers’ to dispose of the ‘corpse’, with the process of antigen presentation being inhibited by rapid removal of DAMPs and tumor debris, which induce immune tolerance [39]. It is worth noting that the ‘corpse’ of the tumor can also cause the ‘pollution’ of TME. For example, potassium ions released after tumor cell death can inhibit the antitumor effect of effector T cells (Teff) [40]. Released prostaglandin E2 (PGE2) can also inhibit the activation of DAMPs on macrophages and dendritic cells (DCs) [41]. Similarly, HMGB1 released after iron death of tumor cells induces polarization of M2-type macrophages by binding to AGE [42]. Besides, the high expression of CD39 and CD73 in TME can convert immune-activated ATP into immunosuppressive adenosine, thus forming a negative feedback mechanism of adenosine energy axis conducive to tumor development [43]. On the contrary, the death of the; siblings’ can cause other tumor cells to be alert and enter a ‘state of combat readiness’. Similar to innate immune cells, tumor cells can also express PRRs such as toll-like receptors (TLRs) and P2 × 4 purinergic receptors to recognize DAMPs molecules such as ATP. Then they can activate signaling pathways such as NF-κB and mitogen-activated protein kinase (MAPK), which promote tumor cell proliferation and resist death. What’s more, it induces the production of various inflammatory factors and chemokines to regulate the differentiation and recruitment of immune cells [44].
Biomarkers of tumor innate immunity
The role of innate immunity can be roughly divided into two categories. One is to attack tumor cells directly through phagocytosis, including natural killer cells (NK cells) and macrophages. The other group activates the second line of defense against tumors, namely adaptive immunity, mainly involving DCs through antigen presentation [45]. In order to counter the innate immune response, tumor cells can directly affect the camp of innate immune cells, the bone marrow, by inhibiting the maturation of DCs, granulocytes, and macrophages to eventually form and recruit immunosuppressive myeloid-derived suppressor cells (MDSCs). MDSCs can be classified as granulocyte/polymorphonuclear cells MDSCs (PMN-MDSCs, CD11b + CD14-CD15+/CD66b+) and monocyte MDSCs (M-MDSCs, CD14 + CD15-HLA-DRlo/-) [46]. It is worth noting that the recent study has also found tumors can induce CD45 + erythroid progenitor cells (EPCs) to transdifferentiate into erythroid derived myeloid cells (EDMCs), thereby supplementing MDSCs at the source [47].
Build the first line of defense against tumor
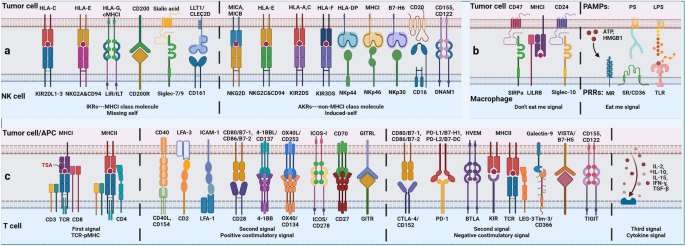
NK cells are native cells with surface markers CD3-CD19-CD56 + CD16+ (CD3 is a marker for T cells and CD19 is a marker for B cells) and intracellular transcription factor E4BP4+. According to the difference of CD56 expression, NK cells can be divided into CD56dim, which mainly plays a cytotoxic role, and CD56bright, which mainly plays an immunomodulatory role [48]. Like T cells, subtypes such as regulatory NK cells, NK cell depletion, and tissue-resident NK cells have been identified [49]. Given the significant heterogeneity of NK cell infiltration in tumors, NK cell frequency predicts the efficacy of immunotherapies such as anti-programmed death receptor-1 (PD-1) [50]. Unlike Teff, NK cells do not express specific antigen-recognition receptors, but regulate activation state primarily through the balance of a series of activated killer cell receptors (AKRs) and inhibitory killer cell receptors (IKRs) signals (Figs. 1 and 2). NK cells do not require antigen presentation and have a broader spectrum, rapid, and safe (for allotransplantation) antitumor effect [51]. Therefore, AKRs and IKRs can be used as NK cell-associated immune checkpoint therapy (ICT) markers.
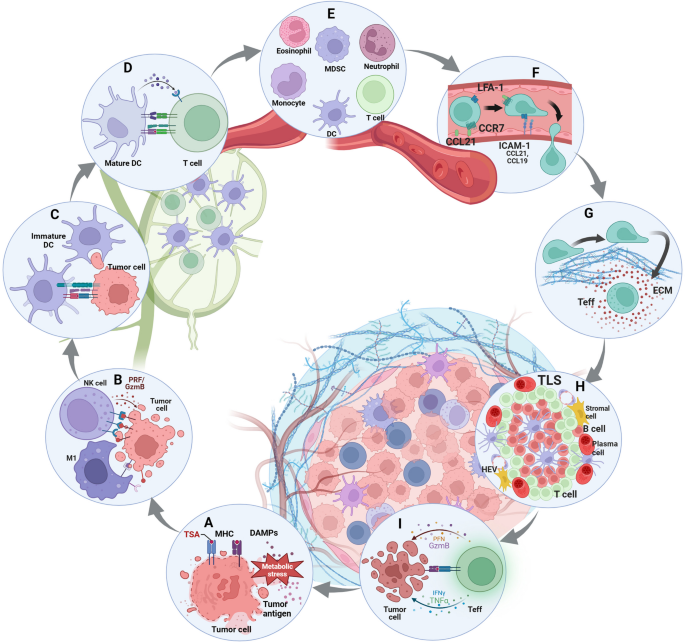
Various links in the development of tumor immunity. Initiation of the tumor immune response: A induce tumor cell ICD to release tumor antigens or promote tumor cell surface expression of pMHC to enhance immunogenicity. Building the first line of defense against tumors: B antitumor innate immune responses, including NK cells and macrophages, kill tumor cells through a balance of inhibitory and activating signals. Activated the second line of anti-tumor defense: C DC captured tumor antigens and migrated to TDLN and gradually matured. D DCs present antigen to T cells. The march of immune cells into the tumor stronghold: E chemokine-regulated immune recruitment in the peripheral blood circulation. F permeable malformed tumor neovascularization. G penetrate the tumor ECM with a rigid structure and immunosuppressive properties. Immune cells form the provisional command of the front line: H the formation of TLS. T cells exert effect: I T cells kill tumor cells through a balance of three signals
Balance between inhibitory and activation of cell-surface immune checkpoints. Anti-tumor innate immunity: NK cells regulate their activation state mainly through the balance of a series of activating killer cell receptors (AKRs, mainly recognizing non-MHCI molecules) and inhibitory killer cell receptors (IKRs, mainly recognizing MHCI molecules). Macrophages regulate their activation state mainly through the balance of a series of ‘eat me’ (DAMPs) and ‘don’t eat me’ (SIRPa) signals. Anti-tumor adaptive immunity: T cells regulate their activation state mainly through the balance of a series of TCR-pMHC, costimulatory molecules and cytokine signaling
In general, non-MHCI molecules on the tumor surface activate NK cells after binding with AKRs while MHCI molecules suppress NK cells after binding with IKRs, but MHCI molecules can also activate AKRs [52]. In order to avoid the recognition of Teff, tumor cells usually down-regulate the expression of MHCI or have gene mutations of human leukocyte antigen (HLA) and β2 microglobulin (β2m). The loss of MHCI will mediate the killing of NK cells, that is, the ‘missing self’ effect, but that process can be disrupted. Due to the influence of TME, the number of NK cells infiltrated in the tumor was less, especially the subgroups with cytotoxic effects [53]. In addition, tumor cells can regulate the balance of AKRs and IKRs to keep NK cells in an inhibited state. For example, the non-classical HLA gene HLA-E has been shown to be highly expressed in various tumors and can provide inhibitory signals to NK cells and CD8 + αβT cells expressing NKG2A/CD94 [54].
Macrophages in tumors are mainly derived from circulating monocytes and tissue-resident macrophages, both of which form tumor-associated macrophages (TAMs) under the influence of TME [55]. In the early stage of immune response, like pro-inflammatory M1 type, TAM mainly plays a killing role, with high expression of CD80, CD86, MHCII, inducible nitric-oxide synthase (iNOS) and CD68 and is dependent on glycolysis. In the later stage of immune response, like anti-inflammatory M2 type, TAM mainly plays an immunosuppressive role, highly expressing CD206, CD204, vascular endothelial growth factor (VEGF), CD163 and arginase (Arg-1), and relying on fatty acid oxidation (FAO) [56]. The study has shown that cell frequency of TAMs is related to response to tumor immunotherapy [57]. Similar to NK cells, TAM regulates activation state mainly through the balance of a series of ‘eat me’ and ‘don’t eat me’ signals which act as a macrophage-related ICT marker. For example, DAMPs expressed by tumor cells convey a ‘eat me’ signal to macrophages, but MHCI expressed by tumor cells conveys a ‘don’t eat me’ signal [58].
Activate the second line of anti-tumor defense
Only DCs can activate initial T cells (Th0) in tumor draining lymph nodes (TDLNs) to initiate an adaptive immune response de novo [59]. Tumor of immature DCs have high expression of PRRs but low expression of MHC, co-stimulatory and adhesion molecules so that they have strong antigen uptake and processing ability but weak ability to present antigen. In the process of migration to TDLN, DCs gradually mature and exhibit receptor expression and function opposite to the immature state [60]. Notably, tumors inhibit the maturation of DC through immunosuppressive TME, specifically tumor extracellular matrix (ECM) related components. For example, the presentation of tumor antigens can be inhibited by mechanisms such as DC-dependent T cell activation induced cell death (AICD) [61]. The maturation of type 1 conventional dendritic cells (cDC1) was found to be interfered with by NF-κB and specific inactivation of interferon regulatory factor 1 (IRF1) [62]. More importantly, tumor-derived immunosuppressive factors (such as transforming growth factor β (TGF-β)) and tumor cells with lymph node metastasis can reshape the TDLN microenvironment and directly affect the production of Teff [63].
Unlike the state of individual cell types, the collection of cells with functional connections, that is, the immune archetype, better reflects the intrinsic connections and interactions of tumor immunity [64]. For example, cDC1 mainly supports the effects of proliferative tumor-antigen specific TCF-1 + CD8 + T cells and is regulated by chemokines secreted by NK cells [65]. Type 2 conventional dendritic cell (cDC2) mainly supports the effects of CD4 + T cells and is inhibited by their derivative subtype regulatory T cells (Tregs) [66]. Contrary to conventional wisdom, the recent study has shown that cDC1 can activate CD4 + T cells through MHCII cross-presentation of tumor antigens. In turn, CD4 + T cells can also promote cDC1 function through CD40 signaling [60]. In addition, a subpopulation of DCs characterized by immunomodulatory genes, namely mature regulatory dendritic cell (mregDC) rich in immunomodulatory molecules, are associated with uptake of dead tumor cells and inhibit the role of cDC1-activated T cells in TDLN [67]. Therefore, all the molecules, cell types and TDLN microenvironment involved in the antigen presentation process of DCs and T cells can be used as biomarkers of immunotherapy.
Biomarkers of tumor adaptive immunity
The strategies of innate and adaptive immunity to recognize tumors are different. Innate immunity mainly recognizes the absence of normal cell biomarkers through the ‘missing self’ effect while adaptive immunity mainly recognizes the appearance of abnormal tumor biomarkers by binding T cell receptors (TCRs) to antigenic peptide-MHC (pMHC) [68]. Among them, tumor adaptive immunity mainly relies on T cells. But for tumor humoral immunity, on the one hand, tumor-specific antibodies can activate complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and antibody-dependent cell-mediated phagocytosis (ADCP) of NK cells and macrophages, and even block some tumor-promoting growth factor receptors [69]. On the other hand, tumor-specific antibodies cannot target internal tumor antigens when the expression level of tumor surface antigens is low [70]. Certain boosting antibodies or tumor-targeting monoclonal antibodies (mAbs) can even inhibit the cytotoxic effect of NK and T cells by promoting the expression of PD-L1 and indoleamine 2, 3-dioxygenase (IDO). They can also promote tumor metastasis by binding to cell adhesion molecules [71].
Immune cells enter the tumor base camp
Although current immunotherapy mainly focuses on remodeling the local TME, more and more studies have begun to focus on the systemic immunity beyond tumors, including TDLN, bone marrow, and peripheral blood circulation. The study has found that the frequency of immune cells and the level of immune molecules in blood circulation can be used as potential markers for immunotherapy [72]. In addition, Teff in TME in response to ICT are mainly derived from the peripheral input of T cell precursors of exhaustion (Tpex) in TDLN rather than from local T cell exhaustion (Tex), indicating that immunotherapy not only regulates the local TME, but also changes the systemic immunity of whole body [73].
With the function of the corresponding chemokines, immune cells are released from the TNDL and bone marrow into the bloodstream and directed to the site of the tumor immune response. But chemokine secretion in TME is often altered [74]. Since different immune cell subpopulation has different chemokine receptor expression patterns, abnormal expression of chemokines may regulate the differentiation, recruitment, homing and recycling of lymphocytes according to the needs of tumor survival or therapeutic intervention [75]. In addition, chemokines are involved in tumor angiogenesis, proliferation, and invasiveness by directly targeting non-immune cells in TME, such as tumor, stromal, and vascular endothelial cells. Therefore, chemokines are important biomarkers of tumor immunity and valuable therapeutic targets [76].
However, the process by which immune cells infiltrate tumor is hindered in many ways. The first barrier that immune cells encounter in the circulation of the blood is the endovascular glycocalyx formed by the ECM. The malformed tumor neovascularization system prevents the immune cells from entering the tumor [77]. Depletion of the Rgs gene has been found to normalize tumor vasculature, thereby promoting immune cell infiltration [78]. Different from the classical endothelium-dependent tumor angiogenesis pathway, tumor vasculogenic mimicry (VM) is a novel tumor microcirculation model independent of the body’s endothelial cells. The vascular pathway is constructed by the tumor cells themselves. Although there is a lack of direct studies to prove the effect of VM on tumor immunity, it can be speculated that the absence of endothelial cells impedes the endothelium-dependent adhesion and migration of immune cells, thereby hindering immune infiltration and distribution within the tumor. More importantly, rigid structure and immunosuppressive tumor ECM, especially collagen secreted by cancer-associated fibroblasts (CAFs), not only hinder the infiltration of immune cells. They also reduce the release of tumor antigens, interfere with antigen presentation and affect the effect of T cells, and even prevent the penetration of anti-tumor drugs [79]. However, ECM, as a protective shell of the tumor, can also hinder the outward progression and metastasis of the tumor, so the dissolution of ECM is one of the signs of tumor metastasis [80].
Immune cells form a temporary command line
In order to combat the immunosuppressive TME, immune cells in tumors do not fight alone but form a systematic team battle and intratumoral immunity cycle. This is evident in the formation of a densely populated area with MHCII high expression APC and CD8 + T cells, known as the APC niche, based on the immune archetype [81]. They may also form more types of lymphoid structures that regularly gather immune cells, known as TLS. The APC niche and TLS not only reflect the number and location of immune cell infiltration in the tumor, but also reflect the heterogeneity of its spatial distribution and the interaction between functions [82]. The study has found that the APC niche is associated with producing an effective and long-lasting immunotherapy response, but it is unclear whether the APC niche is an early stage of TLS. Similarly, the study has found that tumor-specific CD8 + T cells aggregated in TLS are rarely functionally exhausted and exhibit typical memory characteristics [83]. The study has also found that TLS mediates B cell maturation, plasma cell differentiation, and antibody formation [84]. Therefore, the development of TLS is associated with improved ICT efficacy, which can enhance immune infiltration and effect, and generate anti-tumor immune sites [81]. . Therefore, the APC niche and TLS are important markers for immunotherapy. However, TLS is also affected by tumor-produced cytokines and metabolic factors, such as TLS-associated Tregs, regulatory B cells (Bregs), interleukin-10 (IL-10), and enhanced antibodies, which lead to the formation of immunosuppressive TLS and macrophage or NK-cell-dependent apoptosis [85].
Process and outcome of T cell effect
Diffrent from the classical mode of activation for acute bacterial or viral infections, T cell activation of tumor can be divided into two stages: initial activation of cDC antigen presentation in TDLN and subsequent effector activation within the tumor [86]. Among them, effector activation can be roughly divided into three signals: TCR-pMHC, co-stimulatory molecules and cytokines [86]. Interestingly, the effect of T cells is not limited to single one cell, but is accumulated with the number of cells and the duration of action, that is, additive cytotoxicity. Particularly in solid tumors, sublethal injury events delivered by multiple T cells to a single tumor cell mediate effective tumor killing through time-dependent integration [87].
Most current studies focus on CD8 + T cells and tumor-constitutionally expressed MHCI, but the cytotoxic effects of CD4 + T cells and tumor-induced expression of MHCII also deserve our attention. The study has shown that interferon-γ (IFN-γ) induces tumor cells to express MHCII through MHC class II transactivator (CIITA), which is recognized and killed by cytotoxic CD4 + T lymphocyte (CD4 + CTL) [88]. Among them, class I MHC-restricted T-cell-associated molecules (CRTAM) can be used as early biomarkers of CD4 + CTL [89]. The effects of CD4 + CTL require external stimulation to be maintained [90]. Unlike MHCI, MHCII can bind to a higher diversity of antigenic peptides, thereby increasing the likelihood of CD4 + CTL recognition of TSA [88]. More importantly, the study found that MHCI and MHCII are independently regulated in tumor immunity, so their expression may have independent implications for immunotherapy [91].
Tumor cells can evade T-cells’ killing function in a variety of ways. In TDLN, immature DCs, upregulated TGF-β and down-regulated IL-2 can maintain T cells in quiescence. The transcription factor FOXO1 and its induced Kruppel-like factor 2 (KLF2) as well as V-domain Ig suppressor of T cell activation (VISTA) were highly expressed in static T cells [92]. However, tumor cells downregulate the expression of MHCI to induce the loss of TCR-pMHC signal, thus keeping T cells ignorant. Unlike quiescence, ignoring T cells can not specifically recognize tumor antigens [93]. However, even if the TCR-pMHC signal can be activated, the tumor can still maintain T cell anergy by downregulating positive co-stimulatory molecules [94]. The expression of IL-12, IFN-γ and tumor necrosis factor (TNF) was significantly decreased by anergic T cells. Only in the absence of tumor antigen stimulation can anergic T cells gradually resume their functional response [95]. However, interestingly, even in the absence of external activation from tumor cells, T cells can activate CD28 costimulatory signals by cis-B7:CD28 interactions at invaginated synapticmembranes, that is autologous signaling, thereby enhancing their ability to attack tumors [96].
In addition to down-regulating positive costimulatory molecules, tumor cells can also up-regulate the expression of negative costimulatory molecules. This helps maintain T cells in a state of depletion under the influence of stimulatory factors in TME such as chronic TCR signaling [97]. Genetic analysis revealed extensive chromatin remodeling and a close correlation with transcriptional regulatory factor TOX in Tex [98]. Although Tex overlaps with anergic T cells in the expression of inhibitory receptors, anergic T cells appear primarily early in immune response while Tex is produced late from Teff [99]. Based on the expression of Ly108 (representing transcription factor TCF1) and CD69, Tex is divided into four stages, namely, T cell exhaustion progenitors 1 (TexProg1), T cell exhaustion progenitors 2 (TexProg2), T cell exhaustion intermediate (TexInt) and T cell exhaustion terminally (TexTerm). Starting from TexInt, Tex gradually loses the expression of TCF1, so anti-PD-1 treatment can restore the function of Tex in the first three stages, which may be related to the fact that Tex has not completed the secondary epigenetic regulation [100]. However, not all T cells will be exhausted and lose function. The study has found that some T cells can be pluripotent and regenerative after depletion, that is Tpex. Unlike Tex, Tpex can express CD62L, TCF1, ID3, CXCR5, Ly108 and the transcription factor MYB [101]. Tpex is essential to maintain the Tex library and facilitate the response of ICT. Further study showed that cDC1 insulates Tpex from persistent tumor antigens through the action of pMHCI-TCR to prevent its further depletion [102]. TGF-β has also been shown to be closely related to the immune signal of depletion [103].
In addition, activated T cells can also be induced to develop AICD (peripheral deletional tolerance/death) in the absence of growth factors (such as IL-2) by apoptosis factors such as FasL, TNF and TNF-related apoptosis-inducing ligand (TRAIL) in TME [104]. It was found that the mutual inhibition of long non-coding RNA (lncRNA) NKILA and nuclear foctor-κB (NF-κB) can regulate the sensitivity of T cells to AICD [105]. In conclusion, multi-dimensional integrated T cell profiling, including TCR antigen specificity, sequence analysis, T cell phenotype, activation/exhaustion state, and clonal proliferation, is essential for the monitoring of T cell-based cancer immunotherapy [106].
Emerging important factors affecting tumor immunity
Competition for resource supply: metabolism-related biomarkers
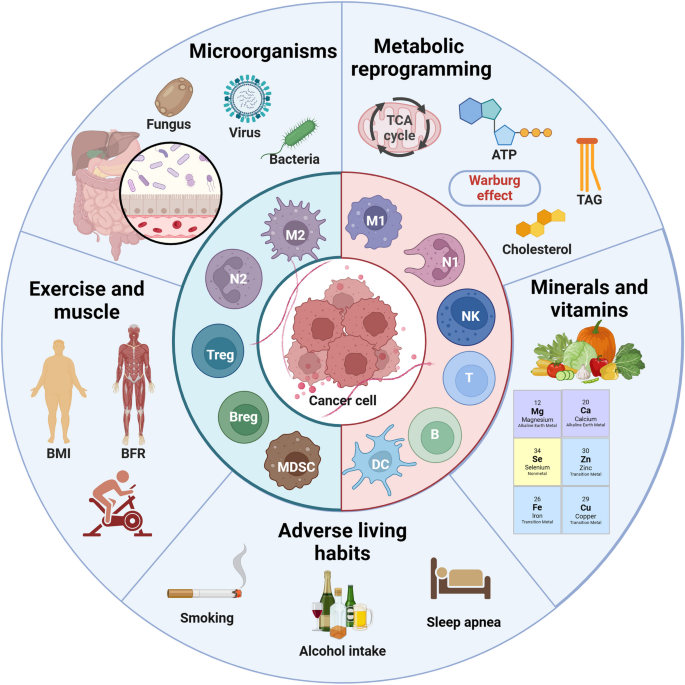
Tumor and immune cells adapt to various stress challenges in TME, such as hypoxia, nutritional competition (deficiency of glucose and key amino acids), acidic environment, and oxidative stress (lipid peroxidation), by changing their own metabolic patterns, that is, metabolic reprogramming [107]. The enhancement of aerobic glycolysis is an important sign of T cell activation and anti-tumor effect. But tumor cells rely on anaerobic glycolysis, that is “Warburg effect”, leading to cell competition for glucose [108]. In addition, tumors of non-gluconeogenic tissues, except for liver and kidney cancer, are able to compensate for glucose deficiency through partial activation of the gluconeogenic pathway [109]. In addition to being an alternative energy source, enhanced lipid metabolism promotes tumor invasion and metastasis, which is usually manifested as an increase in the proportion of unsaturated fatty acids (especially monounsaturated fatty acids) on the cell membrane and the accumulation of intracellular cholesterol. However, lipid metabolism promotes the transformation of immune cells to a protumor phenotype and inhibits the function of anti-tumor immune cells [110]. Similarly, tumor cells and immune cells compete for key amino acids, such as glutamine (Gln), which is essential for cell proliferation, metabolite production, and fatty acid synthesis [111]. In addition, the overexpression of IDO in tumor cells promotes the essential amino acid tryptophan (Trp) to catalyze kynurenine (Kyn). It leads to both the suppression of anti-tumor T cells caused by Trp deficiency and the activation of tumor-promoting immune cells mediated by Kyn accumulation [112]. Notably, some metabolism-regulating drugs can be used as concomitant drugs to promote tumor immunotherapy. For example, statins inhibit their ability to stabilize the expression of PD-1/PD-L1 on the cell membrane by blocking the synthesis of cholesterol [113]. In addition, metformin promoted the transformation of TME to an antitumor phenotype by increasing the number of CD8 + T cells while decreasing the expression of M2 macrophages (Fig. 3) [114].
Important off-field factors affecting tumor immunity. In addition to tumor immunity itself, metabolic reprogramming (especially lipid metabolism) of tumor and related immune cells, as well as specific trace elements and vitamins affect the energy competition between cells at the metabolic level. As a new field of tumor immunity, tumor microbes and the microenvironment they create affect various aspects of tumor immunity, such as tumor immunogenicity, antigen presentation, ICD, metabolism, and ICs. In addition, exercise and bad habits also play an auxiliary role in tumor immune response that cannot be ignored. The above factors can be used as potential targets for regulating TME and improving immunotherapy
Supply of resource supplements: trace element and vitamin related biomarkers
Nutritional status is closely related to immune homeostasis, especially for cancer which is a wasting disease. The changes in the levels of related trace elements and vitamins in the body will affect the tumor immune response [115]. Carcinogenic trace elements are mostly heavy metal elements, such as arsenic, chromium, nickel, beryllium, cadmium and lead, while anti-cancer trace elements, such as selenium, magnesium, zinc, molybdenum, iron, calcium and copper. For example, selenium compounds protect cell-membrane structures and promote antitumor immune responses, primarily through antioxidant effects, particularly the clearance of lipid peroxides [116]. Similarly, Ca²+, Fe²+ and Cu²+ also affect the REDOX balance and mediate Ca²+ overload, ferroptosis and cuproptosis of tumor cells to activate anti-tumor immune responses [110]. In addition, zinc also promotes lipid metabolism and decreases cellular lipid supply and accumulation [117]. It is worth noting that trace elements have a relatively strict proportion in the body. High or low content or improper proportion of each element will affect tumor immunity. For example, excessive iron will also lead to oxidative stress of immune cells, resulting in ferroptosis and inhibition of immune function [118]. In addition to trace elements, vitamins also affect tumor immunity. For example, high-dose vitamin C can increase T-cell infiltration and improve the efficacy of ICT [119]. Similarly, dietary supplementation with vitamin E could enhance patient response to ICT by increasing tumor antigen presentation and activating T cell-mediated antitumor effects [120]. However, dietary ingredients and nutritional supplements are diverse and complex in composition, and their modulation of antitumor immune response and contribution to immunotherapy remain to be explored.
The third party of tumor immunity: microbial-related biomarkers
The influence of microorganisms on tumors can be roughly divided into two aspects. For one thing, intestinal microorganisms indirectly affect the progression of tumors mainly through metabolic pathways. For example, gastrointestinal cancer and liver cancer may be regulated by the metabolites of intestinal microorganisms such as bile acids [121]; In breast cancer, intestinal microorganisms may interfer with steroid metabolism and lead to changes in estrogen spectrum [122]. Even brain tumors are thought to be influenced by gut microbes via the gut-brain axis [123]. For another, the microbiota within tumors has also been shown to influence tumor progression and therapeutic response. For example, increased intratumoral microbiota can lead to mutagenesis in tumorigenesis, modulation of carcinogenic pathways, or alterations in the host immune system [124]. The study has found that intestinal flora can predict the efficacy, change the metabolism of immunotherapy drugs, and affect the bioavailability and efficacy of drugs in the host body [125]. In general, patients with higher diversity and more stable composition of intestinal flora have better ICT efficacy [124]. Besides, opportunistic fungi can also influence the immune response through the fungus-sensing protein Dectin-1. Therefore, inhibiting bacteria may impair tumor immunity while inhibiting fungi may enhance immunotherapy efficacy [126]. However, given the diversity of microbiome and the dynamic changes in microenvironment, as well as the complex links between the microbiome and genetics, environment and diet, the complex interactions between the microbiome and tumor immunity need to be further studied with standardized measures [124].
Auxiliary regulation of tumor immunity: exercise-related biomarkers
Exercise is an important part of comprehensive treatment of cancer and is related to the amount, time, frequency, and mode of exercise. The study found that moderate-to-vigorous intensity aerobic exercise or late morning aerobic exercise was more effective in improving metabolism and lipolysis, while there was no significant difference in the risk of death from cancer between regular and concentrated daily exercise [127]. Of note, the effects of exercise in reducing tumor mortality and delaying tumor progression, which is difficult to reverse existing tumors, are achieved primarily by promoting antitumor immunity [128]. Specifically, exercise enhances the differentiation and recruitment of anti-tumor immune cells by stimulating sympathetic nerves, regulating endocrine levels, accelerating metabolism and even improving mood, which helps relieve immunosuppressive factors such as hypoxia, oxidative stress and high lipid in TME [127]. For example, aerobic exercise can inhibit the growth of pancreatic cancer in mice by activating the immune system, especially IL-15Rα + CD8 + T cells [129]. As an indicator of exercise performance, the body’s muscle mass can be used as a potential marker of tumor immunity, and the study has also considered sarcopenia as a marker of poor prognosis of ICT [130]. The newly generated muscle will secrete many hormones and growth factors during exercise and contraction, which are collectively called myokine [131]. IL-4/6/7/8/15 helps to maintain the balance between pro-inflammatory and anti-inflammatory mediators and promote the differentiation, recruitment and effect of anti-tumor immune cells. In addition, the increase in actin in the circulation caused by exercise helps to activate the immune system [132].
Effect of bad living habits on tumor immunity
Unhealthy lifestyle habits, such as smoking, drinking and staying up late, not only have carcinogenic effects, but also have immunosuppressive effects. Smoking or second hand smoke can lead to a variety of cancers [133]. Interestingly, smokers have a better effect on ICT than non-smokers. The further study has found that cigarette smoke induces the overexpression of PD-L1 through aryl hydrocarbon receptor, which leads to tumor immune escape and sensitivity to ICT [134]. However, some studies have found that smokers’ tumors highly express tissue resident memory T cells (TRM), which mediates immune escape and is insensitive to immunotherapy by exerting immune pressure on tumors [135]. Smoking also raises risk for colorectal cancer (CRC), especially in people with low T-cell responses. Similarly, alcohol use, even in moderation, reduces antitumor immune surveillance by mediating DNA damage (acetaldehyde), affecting the metabolism of micronutrients (e.g., folate, vitamins B12, B6, and A), or generating large amounts of free radicals [136]. In addition, alcohol was found to promote PD-L1 expression on CRC cells through the induction of aldehyde dehydrogenase 2 [137]. Similarly, it has been demonstrated that the disruption of circadian clock caused by regular stay up will greatly destroy the proportion of immune cells and induce immunosuppressive TME, which will significantly reduce the ability of immune cells to destroy cancer cells and promote the rapid growth of tumors [138].
Clinical application of tumor immune biomarkers
Tumor immune biomarkers can be roughly divided into two categories. One is tumor-related biomarkers, mainly tumor antigens, which are used to directly target tumor cells. The other is immune-related biomarkers, mainly immune checkpoints (ICs), which are used to modulate the state of the tumor immune response. Tumor specificity largely determines the clinical application value of tumor immune biomarkers [139].
It is imperative to explore the optimal utilization of biomarkers to enhance their clinical applicability. This encompasses the identification and assessment techniques of markers, the design of drug structures, and the methods of drug delivery. Markers from invasive examination such as biopsy specimens can only reflect the static state of part tumor tissues at a certain point in time due to the heterogeneity of tumor tissues, so the results may be biased or not time-effective [140]. Although the markers from non-invasive examinations such as blood are convenient to dynamically reflect the overall situation of the tumor in real time, the results are difficult to detect or not accurate enough due to low expression level or many other interfering factors [141]. To better detect the expression of PD-L1, new liquid detection methods are being studied, including soluble PD-L1 (sPD-L1) [142], circulating tumor PD-L1 (PD-L1*CTCs) and exosomal PD-L1 [143]. Other liquid detection methods for tumors based on DNA differences in mature red blood cells are also under the research [144]. Different from the traditional “pathological biopsy + immunohistochemistry,” which is subject to the dynamic changes and heterogeneity of the target, immuno-positron emission tomography (immuno-PET) is a novel diagnostic technology that is highly specific, sensitive, non-invasive, and provides real-time dynamic imaging in vivo [145]. By labeling monoclonal antibodies with positron nuclides, immuno-PET combines the targeting specificity of antibodies with the high sensitivity of PET. This approach provides information on tumor immune responses across focal areas, treatment stages, and tumor progression directly by monitoring immune biomarkers such as CTLA-4, PD-1/L1, HER2, and MHC molecules [146, 147].
The drug structure about tumor antigen is mostly similar to antibody drug conjugate (ADC), that is, warhead (such as antibody, TCR or chimeric antigen receptor (CAR) targeting tumor antigen) plus ammunition (such as toxin, drug or immune cells killing tumor cells) and the connection structure of the two. The influencing factors include lysability, solubility, drug antibody coupling ratio (DAR) and bystander effect [148]. The drug structure around ICs is dominated by antibodies, which can be divided into active and blocking types. To increase tumor permeability and reduce immunogenicity, only the Fab segment can be retained, but the Fc segment can mediate the ADCC effect [149].
Regarding drug administration, considering the attributes of chronic tumor diseases and the requirement for prolonged drug usage, the future trajectory for antitumor drugs lies in subcutaneous intratumoral injection or even oral administration (nano-strategies) [150]. Furthermore, the integration of bionic nanoparticles with cell membrane coatings (such as erythrocyte membranes and engineered tumor membranes) enhances the tumor-targeting capability and biocompatibility of drugs, building upon the inherent selective permeability and vascular retention properties of conventional nanomaterials [151]. Similarly, engineered bacteria combined with quorum sensing effects can also help achieve targeted tumor killing [152].
It is important to acknowledge that the combination of different biomarkers can exert better anti-tumor effect. For instance, the combination of ICD inducers and ICT can stimulate immune infiltration while preserving their anti-tumor capabilities [153]. ICIs combined with immune adjuvant, such as IL-15 superagonist, can synergistically enhance anti-tumor immune activation [154]. Additionally, the integration of ICIs with TGF-β antibodies or ECM inhibitors facilitates drug penetration into the tumor [155]. Biomarkers that target both immune and tumor cells can aid immune cells in approaching tumor cells and initiating the cytotoxic effect. The redirection of engineered immune cells by bispecific antibodies (BsAbs) can reverse the off-target and drug resistance of cellular immunotherapy [156].
Treatment strategies based on tumor antigens
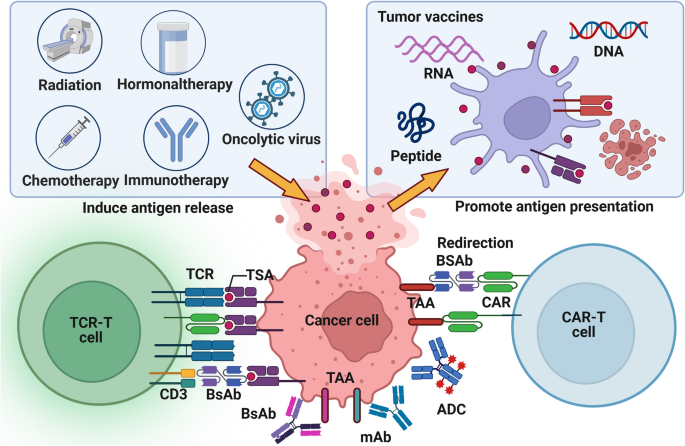
In comparison to TAA, TSA (especially the epitopes) has higher immunogenicity, closer relationship with tumor and more stable expression, so it is not easy to induce immune tolerance, autoimmune response and drug resistance [157]. Therefore, TSA has become a highly potential biomarker in tumor antigen-based therapeutic strategies. Due to the strong heterogeneity of TSA, it is difficult to determine the inevitable relationship between a certain mutation type and TSA, and the number of TSA has been identified is far less and more difficult to apply than TAA [158]. Therefore, we still need to actively search for more TSA shared in tumor patients, such as TSA generated by KRAS G12D2 mutation [159]. An evaluation of the priority of tumor antigens at different expression sites shows that, based on clinical effect, immunogenicity, specificity, tumor correlation, expression level and positive rate, and cell expression distribution, internal tumor antigens (mainly TSA) are more clinically useful than tumor surface antigens and soluble secreted antigens (mainly TAA) [160]. However, it is difficult for most antigen-based treatment strategies to directly target antigens inside tumor cells [158]. Thus, inducing the death of tumor cells contributes to the release of antigens within the tumor and contact with immune cells (Fig. 4).
Tumor antigen-based treatment strategies. Tumor antigen-based therapies encompass tumor vaccines, ACT, tumor antibodies, and inducers of ICD. Tumor vaccines emulate the mechanism through which tumor cells release or express tumor antigens, thereby initiating and augmenting anti-tumor T cell immune responses. These vaccines offer many advantages, including eliciting a broad spectrum of anti-tumor immunity, exhibiting high variability, and demonstrating robust efficacy in overcoming tumor heterogeneity. Compared to established vaccines for infectious diseases, tumor vaccines are relatively straightforward and cost-effective to prepare, except for DC vaccines. However, their utilization in clinical settings remains infrequent. Adoptive cell therapy replicates the procedure of screening and amplifying anti-tumor immune cells, resulting in a substantial enhancement in both quantity and efficacy of these cells. The advantages of ACT in anti-tumor immunity encompass robust and enduring response, prompt initiation, heightened specificity, and potent capacity to overcome immunosuppression. Thereby, ACT circumvents the issue of adverse effects associated with excessive immune stimulation. Nevertheless, certain challenges persist, including limited scope of anti-tumor immune response, diminished variability, inadequate ability to surmount tumor heterogeneity, arduous preparation, protracted process and high cost. Synthetic tumor-targeting antibodies emulate the functional mechanism of naturally occurring antibodies in vivo, thus labeling tumor cells, promoting ADCC effect, blocking tumor-promoting receptor signaling, connecting target cells with killer cells, and carrying anti-tumor substances. Tumor antibodies have the advantages of strong anti-tumor immune responses, high specificity, low side effects and relatively simple preparation, especially for hematological tumors. They account for a large part of the researches and development of anticancer drugs and some of them have entered clinical application. ICD inducers promote tumor production of DAMPs to activate anti-tumor innate and adaptive immune responses. The current clinical application of ICD inducers tends to combine them with immunotherapy or targeted drugs for anti-tumor therapy, and to develop new drug delivery methods
Targeting tumor antigens: tumor surface antigens and MHC-bound internal antigens
The therapeutic strategies targeting tumor antigens, especially TSA, mainly include tumor vaccines, adoptive cell therapy (ACT), and tumor antibodies [158]. For relatively easy-produced and inexpensive tumor vaccines, although the development of preventive vaccines has made significant achievements, most therapeutic vaccines are limited to phase III clinical studies due to low immunogenicity [161]. Therefore, DC vaccines with high immunogenicity and active antigen presentation may be an important direction for future development, but it is still difficult and expensive to produce [162]. Traditional tumor vaccines mainly focus on improving the cellular activity of CD8 + T cells, while the screening of tumor antigen dominant CTL epitope peptides is helpful to find new immune active targets that synergistically activate CD8 + T cells, NK cells and DC [163].
Different from the directly expressed tumor surface antigens, MHC can bind to the intracellular concentrated and processed tumor internal antigens, and express them on the cell surface [164]. Unlike CAR-T cell therapy and tumor antibodies restricted to tumor surface antigens, TCR-T cell therapy utilizes the immune surveillance mechanism to more sensitively recognize a wider range of tumor internal antigens with low levels of variation and thus play a better anti-tumor effect [26]. However, the adaptive regulation of MHC expression level and the individualized restriction of MHC make it difficult for TCR-T to be developed as universal as CAR-T/NK cell therapy, and it is not easy to recognize lipid or carbohydrate tumor-related substances [165]. In addition, both ACT and tumor antibodies are susceptible to the interference of soluble targets and are mistakenly activated in advance (Table 1) [166].
Induction of tumor cell death: release of internal antigens
Unlike the surface antigens of tumors, which are susceptible to the selective pressure of immunotherapy leading to drug resistance, the expression of internal antigens of tumors is more stable and closely associated with the tumor itself [167]. Although stress challenges of TME can spontaneously lead to the death of tumor cells, the artificially induced ICD of tumor cells is more conducive to the release of internal tumor antigens and the activation of immune infiltration [168]. ICD inducers have a dual effect of chemotherapy-immunotherapy. However, he effect of ICD induction in tumor cells by a single ICD inducer is usually weak and limited, which is usually achieved by triggering the secondary or “incidental” effect of anticancer drugs, and even instead promotes the formation of immunosuppressive TME [169]. On the other hand, tumors also evade ICD through various strategies, including the reduction and degradation of ATP release, the reduction of annexin A1 (ANXA1) expression, and the reduction of CRT [170]. Thus, specific interventions that are based on these defective links, such as induction of autophagy, or in combination with other immunotherapies, such as ICIs and ACT, may restore or enhance the efficacy of ICD inducers.
It is necessary to study the effect of tumor mortality and the immune effect of different ways of death [171]. According to morphological, biochemical, immunological, and genetic characteristics, regulatory cell death (RCD) can be divided into apoptotic and non-apoptotic types. Apoptosis is considered immune-tolerated, while non-apoptotic RCD can be classified as autophagy, ferroptosis, pyroptosis, and necroptosis. In some cases, non-apoptotic RCD is considered a manifestation of ICD. On one hand, autophagy can inhibit the immune escape of tumors by degrading immune checkpoints, such as the endocytic recycling of PD-L1 [172]. On the other hand, it can prevent the initiation and activation of anti-tumor T cells by mediating the degradation of MHC class I/II molecules [173]. Ferroptosis has found to enhance the anti-tumor effect of CD8 + T cells [174]. Pyroptosis and necroptosis have been shown to promote DAMP release, trigger antigen presentation, and thus activate adaptive immune responses [175]. Therefore, targeted therapies against autophagy, ferroptosis, pyroptosis, and necroptosis help to convert ICI-resistant “cold” tumors into immunologically active “hot” tumors. However, therapies targeting non-apoptotic RCD may have unintended detrimental effects on tumor-associated immune cells and lead to undesirable toxicity (Table 2) [176].
Treatment strategy based on immune checkpoint
As a crucial regulator of the immune system in healthy individuals, IC plays a pivotal role in preserving autoimmune tolerance and modulating the extent and duration of immune responses. But in the context of tumorigenesis, IC can be exploited by malignant cells, leading to aberrant expression and subsequently facilitating tumor-induced immunosuppression [177]. Therefore, the general policy of IC-based treatment strategy is to induce new or restore the original anti-tumor immune response, but it usually has problems such as low patient response rate, drug resistance and side effects. In addition to taking key IC as the main therapeutic target, other tumor immune biomarkers are also needed to predict and supervise the response of ICT. These include the gene level (such as microsatellite instability (MSI) and TMB) [178], the molecular protein level (such as IL-6, chemokines, inflammation and metabolism-related molecules [179]), the cellular level (such as immune cell frequency, TLS and TDLN), and the microbial level (such as viruses and gut microbes) [180]. In some cases, the efficacy of ICT and its side effects are complementary, that is, good efficacy is often accompanied by side effects, which in turn can be used as a sign of response to treatment [181]. Thus, how to balance ICT and its side effects deserves our further study(Fig. 5).
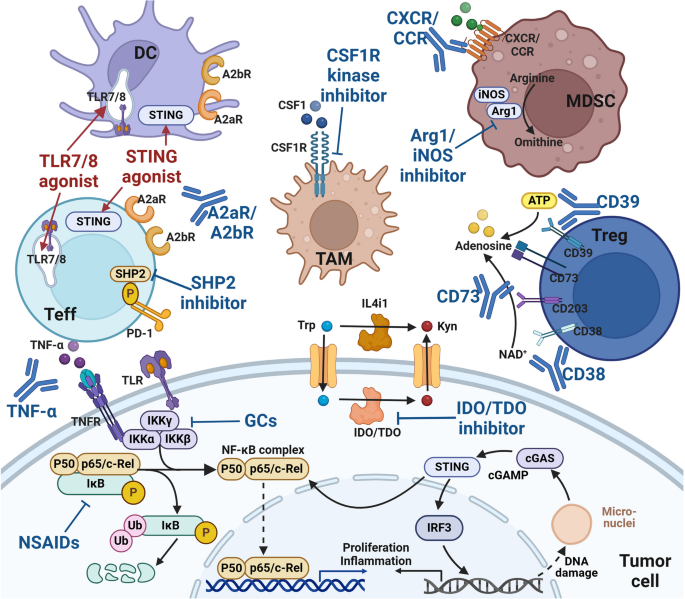
Immune checkpoint-based treatment strategies. Tumor cells actively interact with the surrounding TME and develop various adaptive strategies to form a continuously progressive and highly heterogeneous whole. Various ICs regulate tumor immune responses through a balance of inhibitory and activating signals. In addition to classical cell-surface ICs (such as PD-1/PD-L1 and CTLA-4), they also include extracellular ICs (such as TNF-α and TGF-β), intracellular ICs (such as NF-κB, STING, NR2F6 and LMTK3) and metabolism-related ICs (such as IDO and CD73). More and more ICs pathways have been found to play an important role in driving tumor immune escape. Therefore, ICS pathway may become a promising target for the development of new anticancer immunotherapy
Targeting tumor-local immunity: the activation state of immune cells
A series of ICs that regulate the activation state of immune cells mainly include AKRs and IKRs of NK cells, ‘eat me’ and ‘don’t eat me’ receptors of macrophages, and positive and negative co-stimulatory molecules of T cells. In addition to classic ICs such as PD-1/L1 and CTLA-4, innate immune signals (such as TLR and STING) [182], adenosine axis signals (such as CD39 and CD73) [43], metabolic pathways of key amino acids (such as IDO) and downstream signals of TCR (such as tyrosine protein phosphatase non-receptor types (PTPN6 and PTPN22)) are all involved in the regulation of immune cell activation status [183]. It can be used as the target of novel small molecule drugs. The difference of efficacy and applicable cancer types among different ICs and the interaction between them deserve our attention.
The severity of IRAEs is contingent upon the extent of cellular involvement in ICT expression, the stage of cellular differentiation, and the underlying mechanism of action. For instance, it is noteworthy that red blood cells possess the ability to express the macrophage ‘don’t eat me’ receptor CD47, and the employment of immunotherapy targeting it may result in the development of anemia [184]. Additionally, the impact of anti-CTLA-4 primarily affects Th0 cells, potentially leading to excessive immune activation. But anti-PD-1 primarily influences Teff cells, thereby facilitating the restoration of immune-mediated anti-tumor effects [185]. Certain ICIs exhibit a more intricate mechanism of action, potentially exerting a dual effect on tumor cells. Consequently, solely blocking or enhancing their signals may not yield the desired anti-tumor outcome. For instance, as the tumor progresses, tumor cells themselves may develop tolerance to the inhibition of TGF-β, while immune cells remain suppressed due to the abundant secretion of TGF-β. This immune escape of tumors implies that restoring the sensitivity of tumor cells to TGF-β could prove more beneficial in addressing the challenges encountered in TGF-β antibody therapy [186]. In addition, relevant studies on the endocytosis, recycling, and degradation mechanism of PD-L1 help to make up for the deficiency of PD-L1 antibody therapy (Table 3) [187].
Targeted tumor system immunity: development, differentiation and recruitment of immune cells
Tumor as a systemic disease can remodel function and composition of peripheral immune cells. It is mainly manifested by changes in the frequency of immune cells and their related factors in blood circulation and immune organs, resulting in different responses to immunotherapy in patients with different immune backgrounds [72]. Tumors have the capacity to remodel the microenvironment of immune organs, primarily bone marrow and TDLN, thereby impeding the maturation of immune cells and promoting the development of immunosuppressive phenotypes [188]. Additionally, tumors can modulate the expression of chemokines in the bloodstream, leading to the suppression of anti-tumor immune cells while facilitating the migration of pro-tumor immune cells from immune organs to the TME [189]. In addition to immunotherapy, traditional tumor treatments such as surgery, radiotherapy and chemotherapy can also change the immune background environment of the tumor and affect the therapeutic effect [72]. Therefore, the general principle of therapeutic strategies targeting tumor systemic immunity is to promote the development, differentiation and recruitment of anti-tumor immune cells or inhibit them of pro-tumor immune cells. However, the specificity and accuracy of artificial regulation of tumor systemic immunity are low (except for targeting colony-stimulating factor 1 (CSF-1) [190]). At present, it is mainly used to monitor the progress of tumor immunity and predict the effect of immunotherapy by detecting tumor systemic immunity [191].
Conclusions
In the current review, we comprehensively summarized the war between the immune system and the tumors. As a large battle, ICs provide the necessary trace for the immune system and drugs to accurately attack the tumor. Interestingly, emerging factors significantly affect tumor immunity and thus mediate immunotherapy responses. As a form of physiological intervention, exercise reduces cancer risk and cancer-related morbidity, also re-shape TME via several machineries, should deserve more attentions in future research. Given the complex and diverse regulatory mechanisms of anti-tumor immunity, it is certain that an improved understanding of the unbalance between the immune system and the tumors will provide useful insights into tumorigenicity and may lead to novel clinical applications.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- ACT:
-
Adoptive cell therapy
- ADC:
-
Antibody drug conjugate
- ADCC:
-
Antibody-dependent cell-mediated cytotoxic effect
- ADCP:
-
Antibody-dependent cell-mediated phagocytos
- AICD:
-
Activation induced cell death
- AKR:
-
Activated killer cell receptor
- ANXA1:
-
Annexin A1
- APC:
-
Antigen presenting cell
- Arg-1:
-
Arginase 1
- ATP:
-
Adenosine triphosphate
- Bregs:
-
Regulatory B cells
- BsAbs:
-
Bispecific antibodies
- CAFs:
-
Cancer-associated fibroblasts
- CAR:
-
Cchimeric antigen receptor
- CDC:
-
Ccomplement-dependent cytotoxicity
- cDC1:
-
Type 1 conventional dendritic cells
- cDC2:
-
Type 2 conventional dendritic cells
- CRC:
-
Colorectal cancer
- CRT/CALR:
-
Calreticulin
- CSDE1:
-
Cold shock domain-containing protein E1
- CSF-1:
-
Colony-stimulating factor 1
- CTL:
-
Cytotoxic T lymphocyte
- CTLA-4:
-
Cytotoxic T lymphocyte antigen 4
- DAMPs:
-
Damage-associated molecular patterns
- DAR:
-
Drug antibody coupling ratio
- DCs:
-
Dendritic cells
- ECM:
-
Extracellular matrix
- EDMCs:
-
Erythroid derived myeloid cells
- EPCs:
-
Erythroid progenitor cells
- ER:
-
Endoplasmic reticulum
- FAO:
-
Fatty acid oxidation
- Gln:
-
Glutamine
- Her-2:
-
Human epidermal growth factor receptor 2
- HERVs:
-
Human endogenous retroviruses
- HLA:
-
Human leukocyte antigen
- HMGB1:
-
High mobility group protein 1
- HSP:
-
Heat shock protein
- ICIs:
-
Immune checkpoint inhibitors
- ICs:
-
Immune checkpoints
- ICT:
-
Immune checkpoint therapy
- IDO:
-
Indoleamine 2, 3-dioxygenase
- IFN-γ:
-
Interferon-γ
- IKRs:
-
Inhibitory killer cell receptors
- IL-10:
-
Interleukin-10
- iNOS:
-
Inducible nitric oxide synthase
- IRAEs:
-
Immune-related adverse events
- IRF1:
-
interferon regulatory factor 1
- KLF2:
-
Kruppel-like factor 2
- Kyn:
-
Kynurenine
- lncRNA:
-
Long non-coding RNA
- mAbs:
-
Monoclonal antibodies
- MAPK:
-
Mitogen-activated protein kinase
- MDSCs:
-
Marrow-derived suppressor cells
- MHC:
-
Major histocompatibility complex
- M-MDSCs:
-
Monocyte MDSCs
- mregDC:
-
Mature regulatory dendritic cell
- MSI:
-
Microsatellite instability
- MSLN:
-
Mesothelin
- NK cells:
-
Natural killer cells
- NR2F6:
-
Nuclear receptor subfamily Group 2 F-member 6
- PD-1:
-
Programmed death receptor-1
- PGE2:
-
Prostaglandin E2
- pMHC:
-
Peptide-MHC
- PMN-MDSCs:
-
Polymorphonuclear cells MDSCs
- PRRs:
-
Pattern recognition receptors
- RCD:
-
Regulatory cell death
- SASP:
-
Senescence-associated secretory phenotype
- sPD-L1:
-
Soluble PD-L1
- TAAs:
-
Tumor-associated antigens
- TAMs:
-
Tumor-associated macrophages
- TCRs:
-
T cell receptors
- TDLN:
-
Tumor draining lymph node
- Teff:
-
Effector T cells
- Tex:
-
T cell exhaustion
- TGF-β:
-
Transforming growth factor β
- Th0:
-
Initial T cells
- TLRs:
-
Toll-like receptors
- TLS:
-
Tertiary lymphoid structure
- TMB:
-
Tumor mutation burden
- TME:
-
Tumor microenvironment
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- Tregs:
-
Regulatory T cells
- TRM:
-
Tissue resident memory T cells
- Trp:
-
Tryptophan
- TSAs:
-
Tumor-specific antigens
- VEGF:
-
Vascular endothelial growth factor
- VISTA:
-
V-domain Ig suppressor of T cell activation
- VM:
-
Vasculogenic mimicry
- β2m:
-
β2 microglobulin
References
Helmy KY, Patel SA, Nahas GR, Rameshwar P. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 2013;4(10):1307–20.
Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8(4):7175–80.
O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–67.
Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60.
Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, Qian J, Zhang Y, Chang Q, Zhang X, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–52.
Fu W, Lei C, Wang C, Ma Z, Li T, Lin F, Mao R, Zhao J, Hu S. Synthetic libraries of immune cells displaying a diverse repertoire of chimaeric antigen receptors as a potent cancer immunotherapy. Nat Biomed Eng. 2022;6(7):842–54.
Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suárez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38.
Jelski W, Mroczko B. Molecular and circulating biomarkers of gastric cancer. Int J Mol Sci. 2022;23(14):7588.
Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019.
Pudkasam S, Tangalakis K, Chinlumprasert N, Apostolopoulos V, Stojanovska L. Breast cancer and exercise: the role of adiposity and immune markers. Maturitas. 2017;105:16–22.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–30.
Kalaora S, Nagler A, Nejman D, Alon M, Barbolin C, Barnea E, Ketelaars SLC, Cheng K, Vervier K, Shental N, et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature. 2021;592(7852):138–43.
Lim RJ, Liu B, Krysan K, Dubinett SM. Lung cancer and immunity markers. Cancer Epidemiol Biomarkers Prev. 2020;29(12):2423–30.
Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75.
Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365–86.
Bradley RK, Anczuków O. RNA splicing dysregulation and the hallmarks of cancer. Nat Rev Cancer. 2023;23(3):135–55.
Li R, Di L, Li J, Fan W, Liu Y, Guo W, Liu W, Liu L, Li Q, Chen L, et al. A body map of somatic mutagenesis in morphologically normal human tissues. Nature. 2021;597(7876):398–403.
Liu J, Peng Y, Wei W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022;32(1):30–44.
Zhang Z, Lu M, Qin Y, Gao W, Tao L, Su W, Zhong J. Neoantigen: a new breakthrough in tumor immunotherapy. Front Immunol. 2021;12:672356.
Wang J, Xiu J, Farrell A, Baca Y, Arai H, Battaglin F, Kawanishi N, Soni S, Zhang W, Millstein J, et al. Mutational analysis of microsatellite-stable gastrointestinal cancer with high tumour mutational burden: a retrospective cohort study. Lancet Oncol. 2023;24(2):151–61.
Kumar S, Warrell J, Li S, McGillivray PD, Meyerson W, Salichos L, Harmanci A, Martinez-Fundichely A, Chan CWY, Nielsen MM, et al. Passenger mutations in more than 2,500 Cancer genomes: overall molecular functional impact and consequences. Cell. 2020;180(5):915-927.e916.
Sherman MA, Yaari AU, Priebe O, Dietlein F, Loh PR, Berger B. Genome-wide mapping of somatic mutation rates uncovers drivers of cancer. Nat Biotechnol. 2022;40(11):1634–43.
Burbage M, Rocañín-Arjó A, Baudon B, Arribas YA, Merlotti A, Rookhuizen DC, Heurtebise-Chrétien S, Ye M, Houy A, Burgdorf N, et al. Epigenetically controlled tumor antigens derived from splice junctions between exons and transposable elements. Sci Immunol. 2023;8(80):eabm6360.
Chen F, Zou Z, Du J, Su S, Shao J, Meng F, Yang J, Xu Q, Ding N, Yang Y, et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Invest. 2019;129(5):2056–70.
Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12(1):93.
He J, Xiong X, Yang H, Li D, Liu X, Li S, Liao S, Chen S, Wen X, Yu K, et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response. Cell Res. 2022;32(6):530–42.
Majumder A, Sandhu M, Banerji D, Steri V, Olshen A, Moasser MM. The role of HER2 and HER3 in HER2-amplified cancers beyond breast cancers. Sci Rep. 2021;11(1):9091.
Lowery FJ, Krishna S, Yossef R, Parikh NB, Chatani PD, Zacharakis N, Parkhurst MR, Levin N, Sindiri S, Sachs A, et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022;375(6583):877–84.
Naghavian R, Faigle W, Oldrati P, Wang J, Toussaint NC, Qiu Y, Medici G, Wacker M, Freudenmann LK, Bonté PE, et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature. 2023;617(7962):807–17.
Canale FP, Basso C, Antonini G, Perotti M, Li N, Sokolovska A, Neumann J, James MJ, Geiger S, Jin W, et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021;598(7882):662–6.
Jakobsson J, Vincendeau M. SnapShot: human endogenous retroviruses. Cell. 2022;185(2):400-e400401.
Koch U, Radtke F. Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol. 2011;27:539–62.
Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500.
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–75.
Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, Ruano I, Attolini CS, Prats N, López-Domínguez JA, et al. Cellular senescence is immunogenic and promotes antitumor Immunity. Cancer Discov. 2023;13(2):410–31.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Lv J, Zhou Y, Zhou N, Wang Z, Chen J, Chen H, Wang D, Zhou L, Wei K, Zhang H, et al. Epigenetic modification of CSDE1 locus dictates immune recognition of nascent tumorigenic cells. Sci Transl Med. 2023;15(681):eabq6024.
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, Chen Y, Han B. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7(1):286.
Huang J, Xie Y, Sun X, Zeh HJ 3, Kang R, Lotze MT, Tang D. DAMPs, ageing, and cancer: the ‘DAMP hypothesis.’ Ageing Res Rev. 2015;24(Pt A):3–16.
Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, Palmer DC, Gros A, Yamamoto TN, Patel SJ, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537(7621):539–43.
Cilenti F, Barbiera G, Caronni N, Iodice D, Montaldo E, Barresi S, Lusito E, Cuzzola V, Vittoria FM, Mezzanzanica L, et al. A PGE(2)-MEF2A axis enables context-dependent control of inflammatory gene expression. Immunity. 2021;54(8):1665-1682.e1614.
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–96.
Yegutkin GG, Boison D. ATP and adenosine metabolism in cancer: exploitation for therapeutic gain. Pharmacol Rev. 2022;74(3):797–822.
Man SM, Jenkins BJ. Context-dependent functions of pattern recognition receptors in cancer. Nat Rev Cancer. 2022;22(7):397–413.
Maiorino L, Daßler-Plenker J, Sun L, Egeblad M. Innate immunity and cancer pathophysiology. Annu Rev Pathol. 2022;17:425–57.
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, Shu P, Li D, Wang Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362.
Long H, Jia Q, Wang L, Fang W, Wang Z, Jiang T, Zhou F, Jin Z, Huang J, Zhou L, et al. Tumor-induced erythroid precursor-differentiated myeloid cells mediate immunosuppression and curtail anti-PD-1/PD-L1 treatment efficacy. Cancer Cell. 2022;40(6):674-e693677.
Gunesch JT, Dixon AL, Ebrahim TA, Berrien-Elliott MM, Tatineni S, Kumar T, Hegewisch-Solloa E, Fehniger TA, Mace EM. CD56 regulates human NK cell cytotoxicity through Pyk2. Elife. 2020;9:9.
Merino AM, Kim H, Miller JS, Cichocki F. Unraveling exhaustion in adaptive and conventional NK cells. J Leukoc Biol. 2020;108(4):1361–8.
Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 2020;21(8):835–47.
Tarannum M, Romee R, Shapiro RM. Innovative strategies to improve the clinical application of NK Cell-based immunotherapy. Front Immunol. 2022;13:859177.
Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100.
Bossard C, Bézieau S, Matysiak-Budnik T, Volteau C, Laboisse CL, Jotereau F, Mosnier JF. HLA-E/β2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int J Cancer. 2012;131(4):855–63.
Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, Virasami A, Sebire NJ, Kinsler V, Valdovinos A, et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat Commun. 2019;10(1):2387.
An Y, Yang Q. Tumor-associated macrophage-targeted therapeutics in ovarian cancer. Int J Cancer. 2021;149(1):21–30.
Wang H, Tian T, Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. 2021;22(16):8470.
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47.
DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–82.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24.
Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312.
Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81.
Ghislat G, Cheema AS, Baudoin E, Verthuy C, Ballester PJ, Crozat K, Attaf N, Dong C, Milpied P, Malissen B, et al. NF-κB-dependent IRF1 activation programs cDC1 dendritic cells to drive antitumor immunity. Sci Immunol. 2021;6(61):eabg3570.
du Bois H, Heim TA, Lund AW. Tumor-draining lymph nodes: at the crossroads of metastasis and immunity. Sci Immunol. 2021;6(63):eabg3551.
Combes AJ, Samad B, Tsui J, Chew NW, Yan P, Reeder GC, Kushnoor D, Shen A, Davidson B, Barczak AJ, et al. Discovering dominant tumor immune archetypes in a pan-cancer census. Cell. 2022;185(1):184-203.e119.
Schenkel JM, Herbst RH, Canner D, Li A, Hillman M, Shanahan SL, Gibbons G, Smith OC, Kim JY, Westcott P, et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1(+) CD8(+) T cells in tumor-draining lymph nodes. Immunity. 2021;54(10):2338-2353.e2336.
Im K, Combes AJ, Spitzer MH, Satpathy AT, Krummel MF. Archetypes of checkpoint-responsive immunity. Trends Immunol. 2021;42(11):960–74.
Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J, Chudnovskiy A, Maskey S, Walker L, Finnigan JP, et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580(7802):257–62.
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71.
Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, Chen J, Su F, Liu Q, Song E. Immune checkpoint inhibition overcomes ADCP-Induced Immunosuppression by macrophages. Cell. 2018;175(2):442-457.e423.
Wouters MCA, Nelson BH. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin Cancer Res. 2018;24(24):6125–35.
Laumont CM, Banville AC, Gilardi M, Hollern DP, Nelson BH. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer. 2022;22(7):414–30.
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21(6):345–59.
Connolly KA, Kuchroo M, Venkat A, Khatun A, Wang J, William I, Hornick NI, Fitzgerald BL, Damo M, Kasmani MY, et al. A reservoir of stem-like CD8(+) T cells in the tumor-draining lymph node preserves the ongoing antitumor immune response. Sci Immunol. 2021;6(64):eabg7836.
Märkl F, Huynh D, Endres S, Kobold S. Utilizing chemokines in cancer immunotherapy. Trends Cancer. 2022;8(8):670–82.
Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54(5):859–74.
Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19(4):237–53.
Verginadis II, Avgousti H, Monslow J, Skoufos G, Chinga F, Kim K, Leli NM, Karagounis IV, Bell BI, Velalopoulou A, et al. A stromal integrated stress response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat Cell Biol. 2022;24(6):940–53.
Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Gröne HJ, Hämmerling GJ, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453(7193):410–4.
Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H, Mahadevan KK, Wu CJ, Sugimoto H, Chang CC, et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 2022;40(8):818-834.e819.
Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131.
Munoz-Erazo L, Rhodes JL, Marion VC, Kemp RA. Tertiary lymphoid structures in cancer - considerations for patient prognosis. Cell Mol Immunol. 2020;17(6):570–5.
Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375(6576):eabf9419.
Huang Q, Wu X, Wang Z, Chen X, Wang L, Lu Y, Xiong D, Liu Q, Tian Y, Lin H, et al. The primordial differentiation of tumor-specific memory CD8(+) T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell. 2022;185(22):4049-4066.e4025.
Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–55.
Yang H, Sun B, Ma W, Fan L, Xu K, Jia Y, Xu J, Wang Z, Yao F. Multi-scale characterization of tumor-draining lymph nodes in resectable lung cancer treated with neoadjuvant immune checkpoint inhibitors. EBioMedicine. 2022;84:104265.
Prokhnevska N, Cardenas MA, Valanparambil RM, Sobierajska E, Barwick BG, Jansen C, Reyes Moon A, Gregorova P, delBalzo L, Greenwald R, et al. CD8(+) T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor. Immunity. 2023;56(1):107-124.e105.
Weigelin B, den Boer AT, Wagena E, Broen K, Dolstra H, de Boer RJ, Figdor CG, Textor J, Friedl P. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat Commun. 2021;12(1):5217.
Zeng Z, Gu SS, Ouardaoui N, Tymm C, Yang L, Wong CJ, Li D, Zhang W, Wang X, Weirather JL, et al. Hippo signaling pathway regulates cancer cell-intrinsic MHC-II expression. Cancer Immunol Res. 2022;10(12):1559–69.
Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, Aran D, Ilano A, Pai CS, Rancan C, et al. Intratumoral CD4(+) T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell. 2020;181(7):1612-1625.e1613.
Takeuchi A, Saito T. CD4 CTL, a cytotoxic subset of CD4(+) T cells, their differentiation and function. Front Immunol. 2017;8:194.
Juno JA, van Bockel D, Kent SJ, Kelleher AD, Zaunders JJ, Munier CM. Cytotoxic CD4 T cells-friend or foe during viral infection? Front Immunol. 2017;8:19.
ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, Ceeraz S, Lines JL, Peng C, Carriere C, et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science. 2020;367(6475):eaay0524.
Woodham AW, Yan L, Skeate JG, van der Veen D, Brand HH, Wong MK, Da Silva DM, Kast WM. T cell ignorance is bliss: T cells are not tolerized by Langerhans cells presenting human papillomavirus antigens in the absence of costimulation. Papillomavirus Res. 2016;2:21–30.
Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214–21.
Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49(6):1148-1161.e1147.
Zhao Y, Caron C, Chan YY, Lee CK, Xu X, Zhang J, Masubuchi T, Wu C, Bui JD, Hui E. cis-B7:CD28 interactions at invaginated synaptic membranes provide CD28 co-stimulation and promote CD8(+) T cell function and anti-tumor immunity. Immunity. 2023;56(6):1187-1203.e1112.
ElTanbouly MA, Noelle RJ. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat Rev Immunol. 2021;21(4):257–67.
Ford BR, Vignali PDA, Rittenhouse NL, Scharping NE, Peralta R, Lontos K, Frisch AT, Delgoffe GM, Poholek AC. Tumor microenvironmental signals reshape chromatin landscapes to limit the functional potential of exhausted T cells. Sci Immunol. 2022;7(74):eabj9123.
Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571(7764):211–8.
Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ, et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020;52(5):825-841.e828.
Tsui C, Kretschmer L, Rapelius S, Gabriel SS, Chisanga D, Knöpper K, Utzschneider DT, Nüssing S, Liao Y, Mason T, et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature. 2022;609(7926):354–60.
Dähling S, Mansilla AM, Knöpper K, Grafen A, Utzschneider DT, Ugur M, Whitney PG, Bachem A, Arampatzi P, Imdahl F, et al. Type 1 conventional dendritic cells maintain and guide the differentiation of precursors of exhausted T cells in distinct cellular niches. Immunity. 2022;55(4):656-670.e658.
Xie F, Zhou X, Li H, Su P, Liu S, Li R, Zou J, Wei X, Pan C, Zhang Z, et al. USP8 promotes cancer progression and extracellular vesicle-mediated CD8 + T cell exhaustion by deubiquitinating the TGF-β receptor TβRII. EMBO J. 2022;41(16):e108791.
Cardoso Alves L, Corazza N, Micheau O, Krebs P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J. 2021;288(19):5530–54.
Zhou Q, Zhou L, Qian J, Yuan ZL, Chen ZJ. NKILA inhibition protects retinal pigment epithelium cells from hypoxia by facilitating NFκB activation. Biochem Biophys Res Commun. 2018;503(4):3134–41.
Jiang N, Schonnesen AA, Ma KY. Ushering in integrated T cell repertoire profiling in cancer. Trends Cancer. 2019;5(2):85–94.
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20(1):28.
Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95(7):912–9.
Wang Z, Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5(1):30–45.
Yang K, Wang X, Song C, He Z, Wang R, Xu Y, Jiang G, Wan Y, Mei J, Mao W. The role of lipid metabolic reprogramming in tumor microenvironment. Theranostics. 2023;13(6):1774–808.
Anderson PM, Lalla RV. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients. 2020;12(6):1675.
Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, Kurzrock R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022;110:102461.
Mao W, Cai Y, Chen D, Jiang G, Xu Y, Chen R, Wang F, Wang X, Zheng M, Zhao X, et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight. 2022;7(18):e161940.
Salt IP, Nunes JRC, Fullerton MD. Metformin again? Atheroprotection mediated by macrophage AMPK and ATF1. Cardiovasc Res. 2021;117(5):1233–4.
Mohammadifard N, Humphries KH, Gotay C, Mena-Sánchez G, Salas-Salvadó J, Esmaillzadeh A, Ignaszewski A, Sarrafzadegan N. Trace minerals intake: risks and benefits for cardiovascular health. Crit Rev Food Sci Nutr. 2019;59(8):1334–46.
Ozturk M, Metin M, Altay V, Bhat RA, Ejaz M, Gul A, Unal BT, Hasanuzzaman M, Nibir L, Nahar K, et al. Arsenic and human health: genotoxicity, epigenomic effects, and cancer signaling. Biol Trace Elem Res. 2022;200(3):988–1001.
Skrajnowska D, Bobrowska-Korczak B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients. 2019;11(10):2273.
Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J, Zhang C, Zhou F, Chen J, Zhang L. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun (Lond). 2022;42(2):88–116.
Cimmino L, Neel BG, Aifantis I. Vitamin C in stem cell reprogramming and cancer. Trends Cell Biol. 2018;28(9):698–708.
Yang CS, Luo P, Zeng Z, Wang H, Malafa M, Suh N. Vitamin E and cancer prevention: studies with different forms of tocopherols and tocotrienols. Mol Carcinog. 2020;59(4):365–89.
Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT, Lee IC, Yu-Lun K, Chou SH, Luo JC, Hou MC, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2022;10(6):e004779.
Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–80.
Campbell C, Kandalgaonkar MR, Golonka RM, Yeoh BS, Vijay-Kumar M, Saha P. Crosstalk between gut microbiota and host immunity: impact on inflammation and immunotherapy. Biomedicines. 2023;11(2):294.
Galeano Niño JL, Wu H, LaCourse KD, Kempchinsky AG, Baryiames A, Barber B, Futran N, Houlton J, Sather C, Sicinska E, et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature. 2022;611(7937):810–7.
Si H, Yang Q, Hu H, Ding C, Wang H, Lin X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin Cancer Biol. 2021;70:3–10.
Shiao SL, Kershaw KM, Limon JJ, You S, Yoon J, Ko EY, Guarnerio J, Potdar AA, McGovern DPB, Bose S, et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. 2021;39(9):1202-1213.e1206.
Cao C, Friedenreich CM, Yang L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol. 2022;8(3):395–403.
Lee DH, Rezende LFM, Joh HK, Keum N, Ferrari G, Rey-Lopez JP, Rimm EB, Tabung FK, Giovannucci EL. Long-term leisure-time physical activity intensity and all-cause and cause-specific mortality: a prospective cohort of US adults. Circulation. 2022;146(7):523–34.
Kurz E, Hirsch CA, Dalton T, Shadaloey SA, Khodadadi-Jamayran A, Miller G, Pareek S, Rajaei H, Mohindroo C, Baydogan S, et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell. 2022;40(7):720-737.e725.
Richards SJG, Senadeera SC, Frizelle FA. Sarcopenia, as assessed by Psoas cross-sectional area, is predictive of adverse postoperative outcomes in patients undergoing colorectal cancer surgery. Dis Colon Rectum. 2020;63(6):807–15.
Huang Q, Wu M, Wu X, Zhang Y, Xia Y. Muscle-to-tumor crosstalk: the effect of exercise-induced myokine on cancer progression. Biochim Biophys Acta Rev Cancer. 2022;1877(5):188761.
Kim JS, Wilson RL, Taaffe DR, Galvão DA, Gray E, Newton RU. Myokine expression and tumor-suppressive effect of serum after 12 wk of exercise in prostate cancer patients on ADT. Med Sci Sports Exerc. 2022;54(2):197–205.
Idris S, Baqays A, Isaac A, Chau JKM, Calhoun KH, Seikaly H. The effect of second hand smoke in patients with squamous cell carcinoma of the head and neck. J Otolaryngol Head Neck Surg. 2019;48(1):33.
Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H, Fang WF, Zhou YC, Liang F, Zhang C, et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun. 2019;10(1):1125.
Weeden CE, Gayevskiy V, Marceaux C, Batey D, Tan T, Yokote K, Ribera NT, Clatch A, Christo S, Teh CE, et al. Early immune pressure initiated by tissue-resident memory T cells sculpts tumor evolution in non-small cell lung cancer. Cancer Cell. 2023;41(5):837-852.e836.
Rumgay H, Murphy N, Ferrari P, Soerjomataram I. Alcohol and cancer: epidemiology and biological mechanisms. Nutrients. 2021;13(9):3173.
Zhou X, Xiao Q, Jiang F, Sun J, Wang L, Yu L, Zhou Y, Zhao J, Zhang H, Yuan S, et al. Dissecting the pathogenic effects of smoking and its hallmarks in blood DNA methylation on colorectal cancer risk. Br J Cancer. 2023;129(8):1306–13.
Aiello I, Fedele MLM, Román F, Marpegan L, Caldart C, Chiesa JJ, Golombek DA, Finkielstein CV, Paladino N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci Adv. 2020;6(42):eaaz4530.
Pan Y, Fu Y, Zeng Y, Liu X, Peng Y, Hu C, Deng C, Qiu Z, Zou J, Liu Y, et al. The key to immunotherapy: how to choose better therapeutic biomarkers for patients with non-small cell lung cancer. Biomark Res. 2022;10(1):9.
Vaidyanathan R, Soon RH, Zhang P, Jiang K, Lim CT. Cancer diagnosis: from tumor to liquid biopsy and beyond. Lab Chip. 2018;19(1):11–34.
Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W, Tang XM, Sun F, Lu HM, Deng J, et al. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer. 2022;21(1):25.
Cubillos-Zapata C, Martínez-García M, Campos-Rodríguez F, de la Sánchez M, Nagore E, Martorell-Calatayud A, Hernández Blasco L, Chiner Vives E, Abad-Capa J, Montserrat JM, et al. Soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnoea patients. Eur Respir J. 2019;53(2):1801298.
Ilié M, Szafer-Glusman E, Hofman V, Chamorey E, Lalvée S, Selva E, Leroy S, Marquette CH, Kowanetz M, Hedge P, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29(1):193–9.
Liang N, Jiao Z, Zhang C, Wu Y, Wang T, Li S, Wang Y, Song T, Chen JQ, Liang H, et al. Mature red blood cells contain long DNA fragments and could acquire DNA from lung cancer tissue. Adv Sci (Weinh). 2023;10(7):e2206361.
Menzies AM, Lastoria S. PET imaging for cancer immunotherapy: the immuno-PET. Ann Oncol. 2022;33(1):13–4.
Sharma G, Braga MC, Da Pieve C, Szopa W, Starzetz T, Plate KH, Kaspera W, Kramer-Marek G. Immuno-PET imaging of tumour PD-L1 expression in glioblastoma. Cancers (Basel). 2023;15(12):3131.
Rothlauf PW, Li Z, Pishesha N, Xie YJ, Woodham AW, Bousbaine D, Kolifrath SC, Verschoor VL, Ploegh HL. Noninvasive immuno-PET imaging of CD8(+) T cell behavior in influenza A virus-infected mice. Front Immunol. 2021;12:777739.
Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93.
Iizuka A, Nonomura C, Ashizawa T, Kondou R, Ohshima K, Sugino T, Mitsuya K, Hayashi N, Nakasu Y, Maruyama K, et al. A T-cell-engaging B7-H4/CD3-bispecific fab-scFv antibody targets human breast cancer. Clin Cancer Res. 2019;25(9):2925–34.
Quadir SS, Saharan V, Choudhary D, Harish, Jain CP, Joshi G. Nano-strategies as oral drug delivery platforms for treatment of cancer: challenges and future perspectives. AAPS PharmSciTech. 2022;23(5):152.
Yin T, Diao Z, Blum NT, Qiu L, Ma A, Huang P. Engineering bacteria and bionic bacterial derivatives with nanoparticles for cancer therapy. Small. 2022;18(12):e2104643.
Gurbatri CR, Arpaia N, Danino T. Engineering bacteria as interactive cancer therapies. Science. 2022;378(6622):858–64.
Procureur A, Simonaggio A, Bibault JE, Oudard S, Vano YA. Enhance the Immune checkpoint inhibitors efficacy with radiotherapy induced immunogenic cell death: a comprehensive review and latest developments. Cancers (Basel). 2021;13(4):678.
Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, Miller JS, Farhad M, Anderton K, Lindsey K, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19(5):694–704.
Yi M, Li T, Niu M, Wu Y, Zhao Z, Wu K. TGF-β: a novel predictor and target for anti-PD-1/PD-L1 therapy. Front Immunol. 2022;13:1061394.
Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, Wang L, Weiskopf K, Majeti R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7(5):946–56.
Peri A, Salomon N, Wolf Y, Kreiter S, Diken M, Samuels Y. The landscape of T cell antigens for cancer immunotherapy. Nat Cancer. 2023;4(7):937–54.
Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther. 2023;8(1):9.
Zhang Q, Jia Q, Zhang J, Zhu B. Neoantigens in precision cancer immunotherapy: from identification to clinical applications. Chin Med J (Engl). 2022;135(11):1285–98.
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–37.
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–78.
Linette GP, Carreno BM. On the twentieth anniversary of dendritic cell vaccines - riding the next wave. Cancer Res. 2022;82(6):966–8.
Yu Z, Liu W, He Y, Sun M, Yu J, Jiao X, Han Q, Tang H, Zhang B, Xian Y, et al. HLA-A2.1-restricted ECM1-derived epitope LA through DC cross-activation priming CD8(+) T and NK cells: a novel therapeutic tumour vaccine. J Hematol Oncol. 2021;14(1):71.
Dersh D, Hollý J, Yewdell JW. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat Rev Immunol. 2021;21(2):116–28.
Khawar MB, Sun H. CAR-NK cells: from natural basis to design for kill. Front Immunol. 2021;12:707542.
Poorebrahim M, Mohammadkhani N, Mahmoudi R, Gholizadeh M, Fakhr E, Cid-Arregui A. TCR-like CARs and TCR-CARs targeting neoepitopes: an emerging potential. Cancer Gene Ther. 2021;28(6):581–9.
Leko V, Rosenberg SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 2020;38(4):454–72.
Niu X, Chen L, Li Y, Hu Z, He F. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: perspectives for immunotherapy of SCLC. Semin Cancer Biol. 2022;86(Pt 3):273–85.
Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23(8):4854–65.
Inoue H, Tsutsumi H, Tanaka K, Iwama E, Shiraishi Y, Hirayama A, Nakanishi T, Ando H, Nakajima M, Shinozaki S, et al. Increased plasma levels of damage-associated molecular patterns during systemic anticancer therapy in patients with advanced lung cancer. Transl Lung Cancer Res. 2021;10(6):2475–86.
Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G, Galluzzi L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11(11):1013.
Xie C, Zhou X, Liang C, Li X, Ge M, Chen Y, Yin J, Zhu J, Zhong C. Apatinib triggers autophagic and apoptotic cell death via VEGFR2/STAT3/PD-L1 and ROS/Nrf2/p62 signaling in lung cancer. J Exp Clin Cancer Res. 2021;40(1):266.
Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–5.
Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15(1):174.
Yang F, Xiao Y, Ding JH, Jin X, Ma D, Li DQ, Shi JX, Huang W, Wang YP, Jiang YZ, et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023;35(1):84-e100108.
Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196.
Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–37.
Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, Cipolla CK, Bluth MJ, Chaim J, Al-Ahmadie H, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36(17):1685–94.
Yu Y, Wang S, Su N, Pan S, Tu B, Zhao J, Shen Y, Qiu Q, Liu X, Luan J, et al. Increased circulating levels of CRP and IL-6 and decreased frequencies of T and B lymphocyte subsets are associated with immune-related adverse events during combination therapy with PD-1 inhibitors for liver cancer. Front Oncol. 2022;12:906824.
von Itzstein MS, Khan S, Gerber DE. Investigational biomarkers for checkpoint inhibitor Immune-related adverse event prediction and diagnosis. Clin Chem. 2020;66(6):779–93.
Liu X, Shi Y, Zhang D, Zhou Q, Liu J, Chen M, Xu Y, Zhao J, Zhong W, Wang M. Risk factors for immune-related adverse events: what have we learned and what lies ahead? Biomark Res. 2021;9(1):79.
Li S, Mirlekar B, Johnson BM, Brickey WJ, Wrobel JA, Yang N, Song D, Entwistle S, Tan X, Deng M, et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature. 2022;610(7931):373–80.
Chen J, Zhao X, Yuan Y, Jing JJ. The expression patterns and the diagnostic/prognostic roles of PTPN family members in digestive tract cancers. Cancer Cell Int. 2020;20:238.
Hao Y, Zhou X, Li Y, Li B, Cheng L. The CD47-SIRPα axis is a promising target for cancer immunotherapies. Int Immunopharmacol. 2023;120:110255.
Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–86.
Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21(1):104.
Wang Y, Jia Z, Liang C, He Y, Cong M, Wu Q, Tian P, He D, Miao X, Sun B, et al. MTSS1 curtails lung adenocarcinoma immune evasion by promoting AIP4-mediated PD-L1 monoubiquitination and lysosomal degradation. Cell Discov. 2023;9(1):20.
Yip RKH, Rimes JS, Capaldo BD, Vaillant F, Mouchemore KA, Pal B, Chen Y, Surgenor E, Murphy AJ, Anderson RL, et al. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nat Commun. 2021;12(1):6920.
Bhat AA, Nisar S, Singh M, Ashraf B, Masoodi T, Prasad CP, Sharma A, Maacha S, Karedath T, Hashem S, et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: emerging avenue for targeted therapy. Cancer Commun (Lond). 2022;42(8):689–715.
Wei CY, Zhu MX, Zhang PF, Huang XY, Wan JK, Yao XZ, Hu ZT, Chai XQ, Peng R, Yang X, et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J Hepatol. 2022;77(1):163–76.
Fan W, Wang D, Li G, Xu J, Ren C, Sun Z, Wang Z, Ma W, Zhao Z, Bao Z, et al. A novel chemokine-based signature for prediction of prognosis and therapeutic response in glioma. CNS Neurosci Ther. 2022;28(12):2090–103.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science Foundation of China grants (81972484, 82272667), High-level Innovation Team of Nanjing Medical University Program (JX102GSP201727), The Collaborative Innovation Center for Tumor Individualization Program (JX21817902/008), and Beijing Xisike Clinical Oncology Research Foundation (Y-2019AZZD-00680).
Author information
Authors and Affiliations
Contributions
K.Y., R.L. and J.M. wrote the main manuscript text, K.C. and T.Z prepared figures 1-5 and Y.H. prepared tables 1-3. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, K., Lu, R., Mei, J. et al. The war between the immune system and the tumor - using immune biomarkers as tracers. Biomark Res 12, 51 (2024). https://doi.org/10.1186/s40364-024-00599-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-024-00599-5